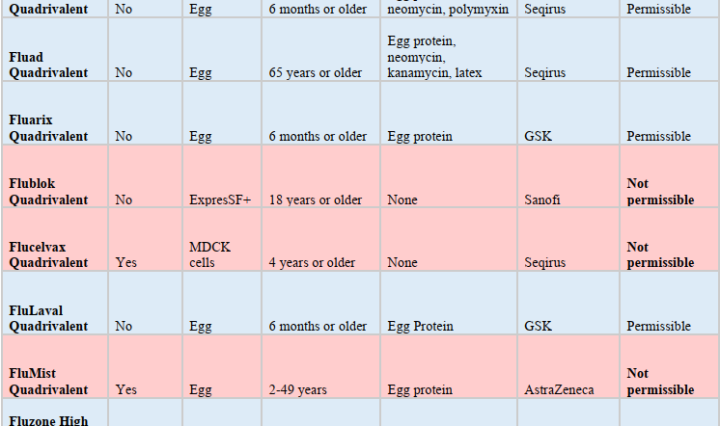
Prepared by Mawlana Dr. Mateen A. Khan, MD and Dr. Ramzan Judge, PharmD Summary Permissible influenza vaccines in the US for 2021-2022 season FluadTM Quadrivalent Fluzone High-DoseTM Quadrivalent AfluriaTM FluarixTM FluLavalTM FluzoneTM Quadrivalent Impermissible influenza vaccines in the US for 2021-2022 season FlublokTM FluMistTM FlucelvaxTM Discussion Background on Influenza Influenza (commonly known as the flu) is a seasonal viral illness carrying significant health, economic, and social burden. For the past nine years, the Centers for Disease Control and Prevention (CDC) estimates that every year 9.3-49 million symptomatic illnesses, 4.3-23 million medical visits, 140,000-960,000 hospitalizations, and 12,000-79,000 deaths are attributed to … Continue reading Islamically Permissible Influenza Vaccines Available in the US for 2021-2022 Season